Kevin Dunleavy, “Novo, Lilly Set to Dominate $71B GLP-1 Drug Market by 2032: J.P. Morgan,” Fierce Pharma, September 11, 2023.
Clara Rodriguez Fernandez, “Competitors Race to Launch GLP-1 Drugs Amid Soaring Weight Loss Demand,” Outsourcing Pharma, October 31, 2024.
Sara Reardon, “How ‘Miracle’ Weight-Loss Drugs Will Change the World,” Nature, November 5, 2024.
Dariush Mozzaffarian, “GLP-1 Agonists for Obesity – A New Recipe for Success?” JAMA, February 29, 2024.
Daniel Drucker, “The GLP-1 Journey: From Discovery Science to Therapeutic Impact,” The Journal of Clinical Investigation, January 16, 2024.
Asher Mullard, “How GLP-1 Went from Being a Hard-to-Handle Hormone to a Blockbuster Success,” Nature Reviews Drug Discovery, November 1, 2024.
Gigen Mammoser, “GLP-1 Drugs like Zepbound and Mounjaro may Help Reduce Sleep Apnea Symptoms,” Healthline, June 21, 2024.
“GLP-1 Drug Liraglutide may Protect Against Dementia,” Alzheimer’s Association, July 30, 2024.
Julia Ries, “Can Ozempic and Other GLP-1 Drugs Reduce Your Dementia Disease Risk?” Healthline, May 12, 2023.
Hugh Waters, Marlon Graf, “America’s Obesity Crisis: The Health and Economic Costs of Excess Weight,” Milken Institute, October 26, 2018.
“Obesity and Overweight,” Center for Disease Control National Center for Health Statistics, October 25, 2024.
“Prevalence of Overweight, Obesity, and Severe Obesity Among Adults Aged 20 and Over: United States, 1960 – 1962 Through 2017 – 2018,” Center for Disease Control National Center for Health Statistics, February 8, 2021.
“Obesity and Severe Obesity Prevalence in Adults: United States, August 2021 – August 2023,” Center for Disease Control National Center for Health Statistics, September 24, 2024.
Clara Rodriguez Fernandez, “Competitors Race to Launch GLP-1 Drugs Amid Soaring Weight Loss Demand,” Outsourcing Pharma, October 31, 2024.
“FDA Approves New Medication for Chronic Weight Management,” U.S. Food & Drug Administration, November 8, 2023.
John P.H. Wilding, et al. “Once-Weekly Semaglutide in Adults With Overweight or Obesity,” The New England Journal of Medicine, February 10, 2021.
Shawn Radcliffe, “3 Million People With Heart Disease May Get Wegovy Medicare Coverage,” Healthline, April 25, 2024.
“FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults With Obesity or Overweight,” U.S. Food & Drug Administration, March 8, 2024.
Regina Castro, “Are There Any Type 2 Diabetes Medication That Can Help People Lose Weight and Lower Their Blood Sugar? Are There Side Effects?” Mayo Clinic, November 14, 2024.
“GLP-1 Agonists,” Cleveland Clinic, July 3, 2023.
Michael Camilleri and Camille Lupianez-Merly, “Effects of GLP-1 and Other Gut Hormone Receptors on the Gastrointestinal Tract and Implications in Clinical Practice,” The American Journal of Gastroenterology, June 2024.
“Semaglutide Shows Promise as a Potential Alcohol Use Disorder Medication,” National Institute on Alcohol Abuse and Alcoholism, March 13, 2024.
Nancy Schimelpfening, “Ozempic, Wegovy May Help Treat Alcohol Use Disorder, Research Shows,” Healthline, November 14, 2024.
Michael Camilleri, Camille Lupianez-Merly, “Effects of GLP-1 and Other Gut Hormone Receptors on the Gastrointestinal Tract and Implications in Clinical Practice,” The American Journal of Gastroenterology, June 2024.
Maurizio Soresi, Lydia Giannitrapani, “Glucagon-Like Peptide 1 Agonists are Potentially Useful Drugs for Treating Metabolic Dysfunction-Associated Steatotic Liver Disease,” National Library of Medicine, August 14, 2024.
Kallirhoe Kalinderi, Vasileios Papaliagkas, Liana Fidani, “GLP-1 Agonists: A New Treatment in Parkinson’s Disease, National Library of Medicine, March 29, 2024.
“Could Drugs Like Ozempic Help People With Parkinson’s Disease?” American Parkinson Disease Association, April 5, 2024.
Liang, Yulin et al., “Clinical Evidence for GLP-1 Receptor Agonists in Alzheimer's Disease: A Systematic Review,” Journal of Alzheimer's disease reports, May 7, 2024.
Corrie Pelc, “GLP-1 Drug Liraglutide May Protect Against Alzheimer’s Disease, Early Trials Say,” Medical News Today, August 2, 2024.
“Targeting the GLP-1/GLP-1R Axis to Treat Osteoarthritis: A New Opportunity?” National Library of Medicine, February 25, 2022.
“GLP-1s and Osteoarthritis,” GLP1 Guide, November 15, 2024.
Kevin Dunleavy, “Novo Nordisk’s Ozempic Cuts Dementia Risk Compared With Merck’s Januvia: Oxford Study,” Fierce Pharma, July 12, 2024.
Milton Packer, et al. “Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity” The New England Journal of Medicine, November 16, 2024.
Gillian McGovern, “Tirzepatide Meets Both Primary End Points in Phase 3 SUMMIT Trial," Pharmacy Times, August 8, 2024.
Regina Castro, “Are There Any Type 2 Diabetes Medication That Can Help People Lose Weight and Lower Their Blood Sugar? Are There Side Effects?” Mayo Clinic, November 14, 2024.
“GLP-1 Agonists,” Cleveland Clinic, July 3, 2023.
Korin Miller, “The FDA is Investigating Potentially Serious Side Effects of Weight Loss Drugs,” Very Well Health, January 11, 2024.
Berkeley Lovelace Jr., “Popular Weight Loss Drugs Links to Rare but Severe Stomach Problems, Study Finds,” NBC News, October 5, 2023.
Brian Deese et al., “Methodological Background for ‘The Miracle Weight-Loss Drug is Also a Major Budgetary Threat,’” New York Times, March 4, 2024.
Julie Hyman, Josh Lipton, “Weight Loss Drugs: Why Congress Needs to Act Fast on Pricing,” Yahoo Finance, March 6, 2024.
“President Biden: Novo Nordisk, Eli Lilly must stop ripping off Americans with high drug prices,” USA Today, July 2, 2024.
All investments are subject to market risk which means their value may fluctuate in response to general economic and market conditions, the prospects of individual companies, and industry sectors due to numerous factors some of which may be unpredictable. Be sure you understand and are able to bear the associated market, liquidity, credit, yield fluctuation and other risks involved in an investment in a particular strategy.
Equity securities are subject to market risk which means their value may fluctuate in response to general economic and market conditions and the perception of individual issuers. Investments in equity securities are generally more volatile than other types of securities. An investment that is concentrated in a specific sector may be subject to a higher degree of market risk.
Global Securities Research (GSR) is a division of Wells Fargo Investment Institute, Inc. (WFII). WFII is a registered investment adviser and wholly owned subsidiary of Wells Fargo Bank, N.A., a bank affiliate of Wells Fargo & Company.
The information in this report was prepared by Global Securities Research (GSR). Opinions represent GSR’s opinion as of the date of this report and are for general information purposes only and are not intended to predict or guarantee the future performance of any individual security, market sector or the markets generally. GSR does not undertake to advise you of any change in its opinions or the information contained in this report. Wells Fargo & Company affiliates may issue reports or have opinions that are inconsistent with, and reach different conclusions from, this report. Past performance is no guarantee of future results.
The information contained herein constitutes general information and is not directed to, designed for, or individually tailored to, any particular investor or potential investor. This report is not intended to be a client-specific suitability or best interest analysis or recommendation, an offer to participate in any investment, or a recommendation to buy, hold or sell securities. Do not use this report as the sole basis for investment decisions. Do not select an asset class or investment product based on performance alone. Consider all relevant information, including your existing portfolio, investment objectives, risk tolerance, liquidity needs and investment time horizon. The material contained herein has been prepared from sources and data we believe to be reliable but we make no guarantee to its accuracy or completeness.
Wells Fargo Advisors is registered with the U.S. Securities and Exchange Commission and the Financial Industry Regulatory Authority, but is not licensed or registered with any financial services regulatory authority outside of the U.S. Non-U.S. residents who maintain U.S.-based financial services account(s) with Wells Fargo Advisors may not be afforded certain protections conferred by legislation and regulations in their country of residence in respect of any investments, investment transactions or communications made with Wells Fargo Advisors.
Wells Fargo Advisors is a trade name used by Wells Fargo Clearing Services, LLC and Wells Fargo Advisors Financial Network, LLC, Members SIPC, separate registered broker-dealers and non-bank affiliates of Wells Fargo & Company.
©2024 Wells Fargo Investment Institute and subject to the CC BY-NC-ND 4.0 DEED. All rights reserved.


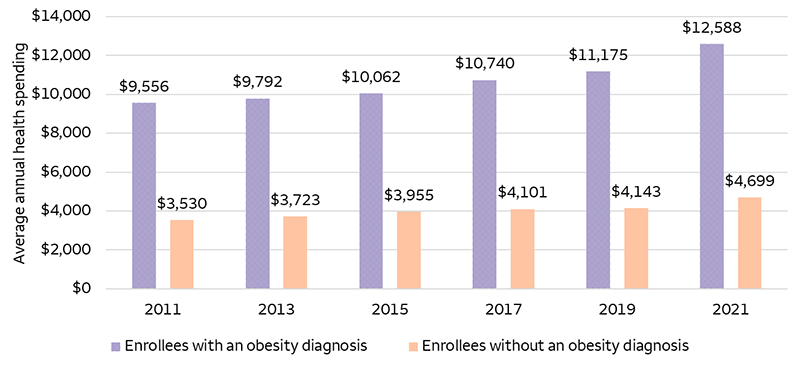 Source: Peterson-KFF Health System Tracker. Data as of July 29, 2024. Includes enrollees with insurance plans from large employers who have a diagnosis of overweight or obesity.
Source: Peterson-KFF Health System Tracker. Data as of July 29, 2024. Includes enrollees with insurance plans from large employers who have a diagnosis of overweight or obesity.